Conjugation of VEGFR1/R2-targeting peptide with gold nanoparticles to boost antiangiogenic and antitumoral exercise | Journal of Nanobiotechnology
Characterization of the synthesized bare and peptide-conjugated GNPs
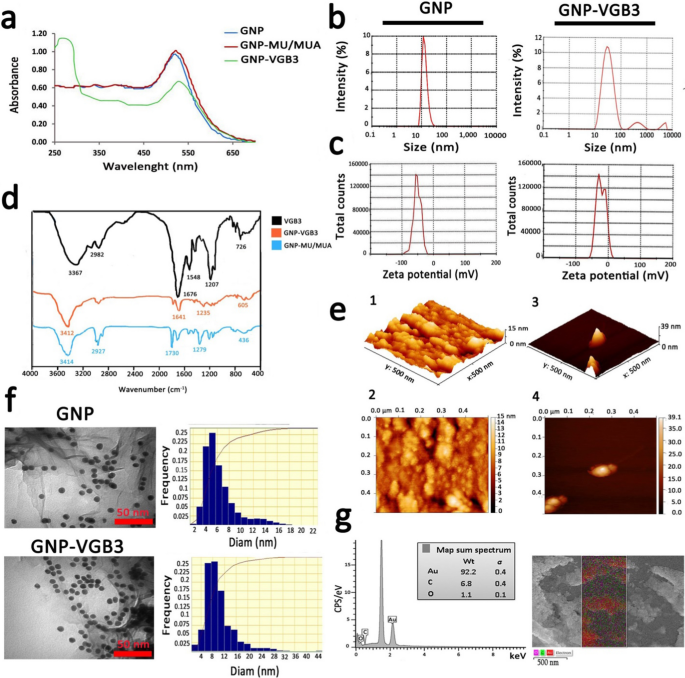
GNPs had been ready as described beforehand [19, 20]. Characterization of GNPs utilizing the UV–Vis spectra (400–700 nm) confirmed an absorption band shift in direction of greater wavelengths (pink shift), indicating the rise of GNP measurement after floor modification. The synthesized GNPs confirmed a robust absorption at λmax ~ 516 nm, which is attributable to globular GNPs with diameters decrease than 20 nm [31, 32]. Addition of MU/MUA to the floor of GNPs led to a notable pink shift from λmax ~ 516 nm to 522 nm. After conjugation of VGB3 to the modified GNPs, a substantial shift from λmax ~ 522 nm to round ~ 550 nm and a brand new single absorption band had been emerged at λmax ~ 280 nm, which the later could be related to the tryptophan residue within the construction peptide (Fig. 2a). Based mostly on measurements by UV customary curve, we now have detected no unconjugated peptide fraction within the response combination.
In response to the DLS graphs (Fig. 2b), the imply diameter of GNPs was 16 nm and the dimensions distribution was between 7–40 nm, whereas the typical diameter of GNP-peptide was 30, indicating a barely extra variation than free GNP. The worth of Zeta potential, an indicator of dispersion stability and the tendency of GNPs to mixture in answer, indicated that GNPs had been negatively charged, which is because of the citrate ions. Immobilization of the positively-charged peptide molecules onto highly-negatively charged (− 51.4 ± 0.88 mv) GNPs decreased the adverse cost to − 23.9 ± 0.55 mv (Fig. 2c).
The characterization of the synthesized bare and conjugated GNPs. a UV–Vis absorption spectra of GNPs earlier than and after modifications. b DLS curves of GNPs and GNP–VGB3 associated to hydrodynamic diameters. c The zeta potentials of GNPs and GNP–VGB3. d FT-IR spectra of GNP and VGB3 and their conjugations within the 400–4000 cm−1 area. e AFM of GNP and GNP–VGB3. (1, 2) AFM evaluation depicted the imply diameter of GNPs in 3D and 2D pictures, respectively (scale bar: 500 nm), and (3, 4) In the identical manner, the obtained pictures from GNP–VGB3 had been devoted inside a scan space of 0.5 μm × 0.5 μm. f TEM pictures to confirm the dimensions, form and the dimensions distributions of GNPs and GNP–VGB3, respectively (scale bar: 50 nm) and g EDS evaluation of GNPs-VGB3 and its mapping to determine every component within the pattern (Au: pink; C: inexperienced, and O: Violet)
Subsequent, the FAAS and ICP-MS evaluation was carried out to attain the gold focus. Accordingly, the Au focus was 48 mg L−1 free of charge GNP and 19 mg L−1 for GNP–VGB3 (information not proven).
The FT-IR spectra allow us to handle the useful teams within the construction of synthesized peptide, MU/MUA modified GNP, and the peptide immobilized on modified GNPs. As indicated in Fig. 2d, VGB3 was bonded from its NH2 group to the –COOH useful teams of MU/MUA modified GNP. In MUA modified GNP, the absorption bands in 3414, 1730 and 1279 cm−1 could be associated to the stretching vibrations of hydroxyl group of COOH, –C=O and –C–O bands within the MU/MUA linker, respectively. The band at 2927 cm−1 was assigned to the stretching vibrations of –C–H in –CH2– teams of MU/MUA. The broad intense absorption band within the wavenumbers of 3367 cm−1 (in VGB3 spectrum) and 3412 cm−1 (in GNP–VGB3 spectrum) was associated to the vibrations of the hydroxyl group of –COOH and –NH teams within the peptide [24]. The stretching vibrations of amidic –C=O band within the peptide had been noticed at 1676 cm−1 (for unconjugated peptide) that overlapped by the vibrations of the carbonyl group of COOH. In each VGB3 and GNP–VGB3 spectra, the stretching vibrations of amidic –NH was occurred in 3150–3350 cm−1 that overlapped with –COOH band and the vibrations of –C–S band was appeared in 600–700 cm−1. The bands in 1207 and 2982 cm−1 could be assigned to the vibrations of –C–N of amid group and –C–H in CH2, respectively. Additionally, the bending vibrations of NH noticed in 1548 cm−1 represented the amide bands. Within the GNP–VGB3 spectra, discount within the band depth of the carbonyl group of COOH in 1730 cm−1 and enhance within the depth of the band at 1641 cm−1 is expounded to amidic –C=O vibrations, indicating the formation of amid band by the covalent linkage between the NH2 group of peptide with –COOH group of MU/MUA conjugated GNP [23, 33].
Determine 2e(1–4) reveals atomic pressure microscopy (AFM) pictures of bare GNP and GNP-peptide inside a scan space of 0.50 μm × 0.50 μm for each samples. In three dimensional pictures, the typical measurement of GNPs and GNP-peptide had been estimated about 15 ± 4 nm and 39 ± 4 nm, respectively [25].
TEM and FESEM had been utilized to specify the morphology of NPs. In response to the outcomes obtained from TEM, the form of free GNPs and GNP-peptide had been spherical with a monodispersed measurement from 5–12 nm (Fig. 2f). The variations within the imply diameters of nanoparticles obtained by TEM and DLS may very well be because of the strategies of measurement measurement. FESEM pictures of GNP-peptide is proven in Further file 1: Fig S2 that displayed floor morphology of this pattern. In comparison with GNP–VGB3 displaying the imply measurement of 25 nm, free GNP displayed a lot bigger measurement, reflecting the susceptibility of free GNPs for aggregation. Based mostly on EDS evaluation, the basic components of the nanoparticles had been the Au component (92.2%), C and O components (6.8 and 1.1%, respectively). The presence of C and O components is attributable to the MU/MUA linkers, and the peptide molecules on the floor of GNPs.
GNP-VGB3 acknowledges and neutralizes VEGFR1 and VEGFR2
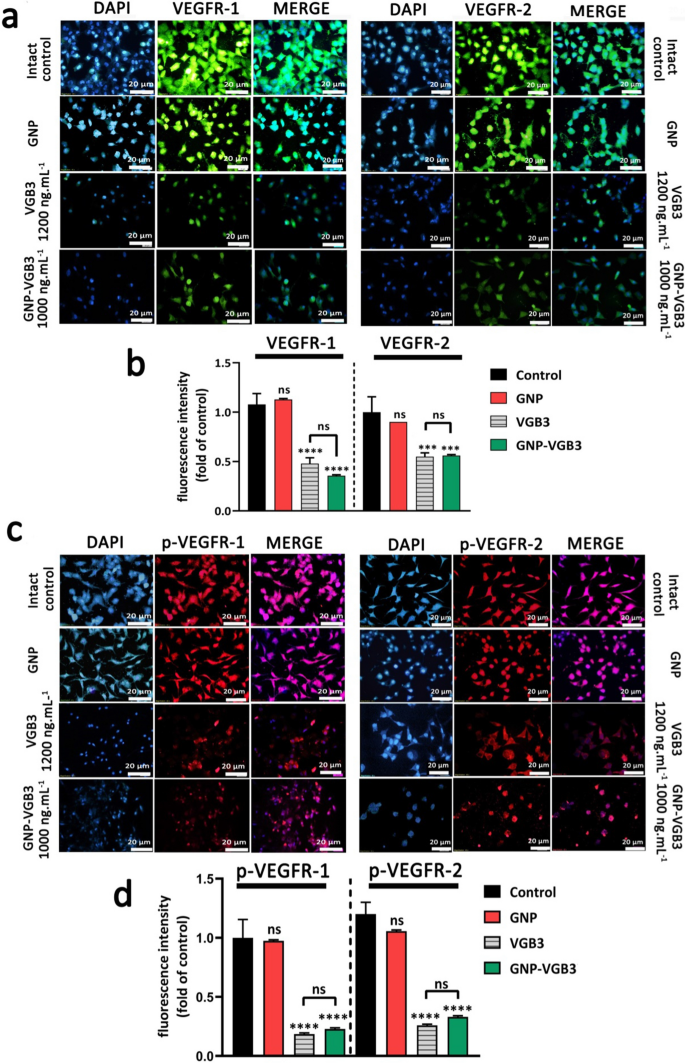
VEGFR2 and VEGFR1 are extremely expressed on the floor of endotheliral cells (ECs) [34]. The precise cell binding of GNP–VGB3 to VEGFR1 and VEGFR2 had been investigated by immunocytochemical assay utilizing human umbilical vein endothelial cells (HUVECs). Pre-incubation of HUVECs by rising concentrations of free VGB3 (500, 700 and 1200 ng mL−1) and GNP–VGB3 (250, 500, 1000 ng mL−1) diminished binding of fluorescently labeled anti-VEGFR1 or anti-VEGFR2 (20 ng mL−1), whereas fluorescence intensities was not affected when HUVECs handled with GNP (Further file 1: Fig. S3). These outcomes point out that VGB3 retained its capability to acknowledge VEGFR1 and VEGFR2 after conjugation to the gold nanoparticles (Fig. 3a, b). As well as, GNP–VGB3 remedy inhibited VEGF-induced phosphorylation of VEGFR2 and VGEFR1. As indicated in Fig. 3c, d, incubation with GNP–VGB3 (1000 ng mL−1) in addition to VGB3 (1200 ng mL−1) diminished fluorescent alerts of anti-phospho VEGFR1 (anti-pVEGFR1) or anti-pVEGFR2 in a dose-dependent method in comparison with controls, whereas binding of the antibodies was not affected by GNP (Further file 1: Fig S4). These outcomes point out that GNP–VGB3 abrogated the VEGF-induced activation (phosphorylation) of VEGFR1 and VEGFR2.
GNP-VGB3 acknowledges VEGFR1 and VEGFR2 and suppresses their VEGF-induced phosphorylation in endothelial cells. a Immunocytochemical pictures of HUVE cells handled with PBS, GNP, VGB3 and GNP–VGB3 utilizing FITC-secondary anti-mouse antibody (inexperienced) to bind to VEGFR1 (left) and VEGFR2 (proper) (scale bar: 20 μm). b Statistical evaluation of VEGFR1/2 fluorescence depth below varied concentrations had been carried out by prism software program 8; Oneway ANOVA and all information displayed imply ± SEM (n = 3). c Immunoflourescent staining pictures (PE-secondary anti-mouse antibody (pink)) of phosphorylated-VGEFR1 (p-VEGFR1) and p-VEGFR2 with varied remedies. d Quantitative evaluation of the fluorescence depth of p-VEGFR1/2 by prism software program 8 analyzed by One-way ANOVA for various remedies. (****P < 0.0001, ***P < 0.001 and NS: not important as compared with management)
Inhibition of proliferation and migration of endothelial and tumor cells
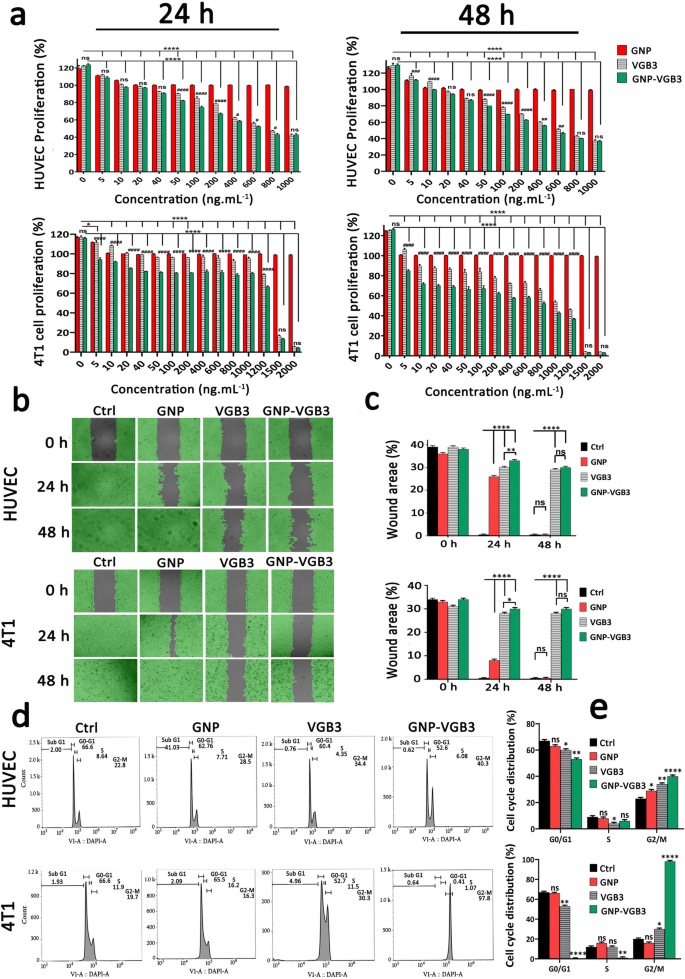
VEGFR1 and VEGFR2 endure dimerization and VEGFR ligand-dependent phosphorylation, which set off mitogenic, chemotactic, and prosurvival alerts, together with stimulation of tumor vessel formation [35]. We’ve beforehand indicated that VGB3 can inhibit proliferation, migration and tube formation of HUVECs, and proliferation of 4T1 mammary carcinoma tumor cells that expresses each VEGFR1 and VEGFR2 [13]. Right here, to verify whether or not VGB3 retained its results in nanoformulation, the antiproliferative and antimigrative properties of GNP–VGB3 had been decided compared to GNP and VGB3 in HUVE and 4T1 cells when stimulated by VEGF (20 ng mL−1). Notably, blank-GNP had no important results on the cell viability and confirmed an analogous consequence to the non-treated cells, inferring that the blank-GNP composition is biocompatible (Fig. 4a). In distinction, VGB3 and GNP–VGB3 exhibited time and dose-dependent cytotoxicity; the half-maximal inhibition (IC50) values of GNP–VGB3 in opposition to HUVECs had been 554 ng mL−1 (24 h) and 440 ng mL−1 (48 h) and free of charge VGB3 had been 710 ng mL−1 (24 h) and 561 ng mL−1 (48 h). Equally, the info of 4T1 cells reveal that the IC50 values of GNP–VGB3 had been 1238 ng mL−1 (24 h) and 423 ng mL−1 (48 h) and free of charge VGB3 had been 1971 ng mL−1 (24 h) and 771 ng mL−1 (48 h). These ends in settlement with earlier research indicated that the cytotoxicity of peptide-conjugated GNP was greater than that of free peptides [36, 37].
Inhibition of HUVE and 4T1 cells proliferation, migration and cell cycle development. a The investigation of cell viability within the HUVECs and 4T1 cells after remedy with completely different concentrations (0–1000 ng mL−1) of GNPs, VGB3, and GNP–VGB3 within the presence of VEGF (20 ng mL−1). Additionally, the cell viability charges of 4T1 cells after remedies with completely different concentrations (0–2000 ng mL−1) of GNPs, VGB3 and GNP–VGB3 after 24 and 48 h incubation. The cell viability charges of handled cells had been evaluated by MTT assay for twenty-four and 48 h incubation utilizing prism software program 8 (One-way ANOVA technique, primarily based on imply ± SEM of six unbiased observations). b HUVE and 4T1 cells wound closure cell migration assay after completely different remedy with PBS, GNPs, VGB3, or GNP–VGB3 within the presence of VEGF (20 ng mL−1) after incubation for 0, 24 or 48 h. By Wimasis picture evaluation, wound areas within the pictures had been proven with a grey colour. c Statistical evaluation of wound space of HUVE and 4T1 cells after remedies by One-way ANOVA, imply ± SD, n = 3). d Cell cycle evaluation of HUVE and 4T1 cells after publicity to PBS, GNPs, VGB3, and GNP–VGB3 and VI stain adopted by circulate cytometry evaluation. e Cell cycle distribution of HUVE and 4T1 handled cells had been analyzed statistically by One-way ANOVA pathway (imply ± SEM, and n = 3, ****P < 0.0001, **P < 0.01, *P < 0.05, or ns: not important)
Cell migration is important for angiogenesis of endothelial cells and invasion of tumor cells. We carried out wound therapeutic assay to research the antimigrative impact of GNP–VGB3 in HUVE and 4T1 cells. Based mostly on the above talked about IC50 values, endothelial cells had been incubated with VGB3 (710 ng mL−1), GNP–VGB3 (554 ng mL−1) for twenty-four and 48 h, and filling the scratch space with cells was evaluated in comparison with controls and GNP-treated cells. When stimulated by VEGF (20 ng mL−1), HUVE and 4T1 cells had been capable of fill the wound space after 24 h. Nonetheless, the VEGF-induced migrations had been inhibited by VGB3 and GNP–VGB3, and to a lesser extent by GNP after 24 h and suppression was maximal in GNP–VGB3-treated teams (P < 0.0001). After 48 h, whereas GNP-treated HUVE and 4T1 cells utterly migrated to the wound space, VGB3 and GNP–VGB3 potently inhibited VEGF-induced migration of cells in comparison with controls and GNP-treated cells (P < 0.0001). Outcomes of wound therapeutic assay indicated that GNP–VGB3 may suppress endothelial and tumor cells locomotion in response to development factor-attractive environment (Fig. 4b, c).
The induction of cell cycle arrest is a method to manage aberrant most cancers cell proliferation [38]. Therefore, to research the mechanism of antiproliferative results, we evaluated the cell cycle distribution utilizing circulate cytometry. Cell cycle evaluation of HUVECs and 4T1 cells uncovered to GNP, VGB3 and GNP–VGB3 is proven in Fig. 4d. In step with the outcomes of proliferation and migration, remedy with VGB3 and GNP–VGB3 resulted in an arrest within the G2/M section, with a major lower in G0/G1 section versus management cells, whereas GNP was ineffective in 4T1 cells or reasonably (P < 0.05) efficient in HUVECs. Importantly, the buildup in G2/M section was considerably greater in HUVE and 4T1 cells handled GNP–VGB3 (40.3 and 97.8%, respectively) than in cells handled with free peptide (34.4 and 30.3%, respectively)(Fig. 4e), suggesting that binding to GNP induced the inhibitory results of VGB3. These outcomes are in line with the info obtained by MTT and scratch analyses and supply additional proof for enhanced efficiency of VGB3 after on account of ligation to GNPs.
Induction of ROS manufacturing and apoptosis in endothelial and tumor cells
Inhibition of VEGF binding to VEGFRs on the endothelial and 4T1 cells ends in apoptosis induction [39]. Alternatively, ROS overproduction can activate the apoptotic signaling pathways and cell demise [40]. We due to this fact evaluated the potential of GNP–VGB3 to induce ROS overproduction adopted and apoptosis induction in VEGF-induced endothelial and tumor cells. First, the intracellular ROS manufacturing was measured in HUVECs and 4T1 cells after remedy with free GNPs, free VGB3, and GNP–VGB3. When handled with concentrations equal to IC50 values and within the presence of VEGF (20 ng mL−1), the fluorescence depth of 2-7-dichlorofluorescin diacetate (DCFDA) as ROS manufacturing probe in response to GNP–VGB3 remedy had been 40.98 and 19.03 in HUVECs and 4T1 cells, respectively, which had been considerably greater than that of GNP (16.11 and eight.00, respectively), free peptide (27.28 and 15.69, respectively) and untreated cells (13.8 and 5.24, respectively) (Fig. 5a, b), indicating that neutralization of VEGF receptors led to the overproduction of ROS.
ROS overproduction and apoptosis induction in HUVE and 4T1 cells. a Intracellular ROS induction of HUVE and 4T1 cells handled with PBS, GNPs, VGB3 and GNP–VGB3 within the presence of VEGF (20 ng mL−1) specified after staining with DCFH-DA by circulate cytometry (scale bar: 20 μm). b Detection of ROS primarily based on fluorescence depth utilizing prism 8.0 software program in HUVE and 4T1 cells after remedies. c Move cytograms of cell apoptosis in HUVE and 4T1 cells induced by PBS, GNPs, VGB3, and GNP–VGB3 within the presence of VEGF (20 ng mL−1) utilizing Annexin V/PI staining. d The entire cell apoptosis in HUVE and 4T1 handled cells had been obtained from the sum of early and late apoptosis, which positioned on the nook of every panel (in lower-right (Annexin V-FITC+, PI−), and upper-right (Annexin V-FITC+, PI+) quadrants, respectively) and represented within the diagram utilizing prism software program. (All information analyzed primarily based on imply ± SEM; One-way ANOVA; n = 3; quantity signal image (#) was used for evaluating the remedies with management, and asterisk image (*) was used for comparability between remedies, ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, or ns: not important)
To check GNP–VGB3-mediated induction of apoptosis, we performed Annexin V and propidium iodide (PI) stainings [41] in VEGF (20 ng mL−1)-induced HUVE and 4T1 cells handled with GNP, VGB3 and GNP–VGB3. As indicated in Fig. 5c and d, the share of apoptotic cells remarkably elevated from 30.98 and 50.83% in GNP- and VGB3-treated cells, respectively, to 74.50% in GNP–VGB3-treated HUVECs. Extra strikingly, the proportion of apoptotic 4T1 cells had been elevated in GNP–VGB3-treated group (82.00%) in comparison with the cells handled by VGB3 (12.35%) and GNP (1.92%) (Fig. 5c, d).
Inhibition of breast tumor development in mice
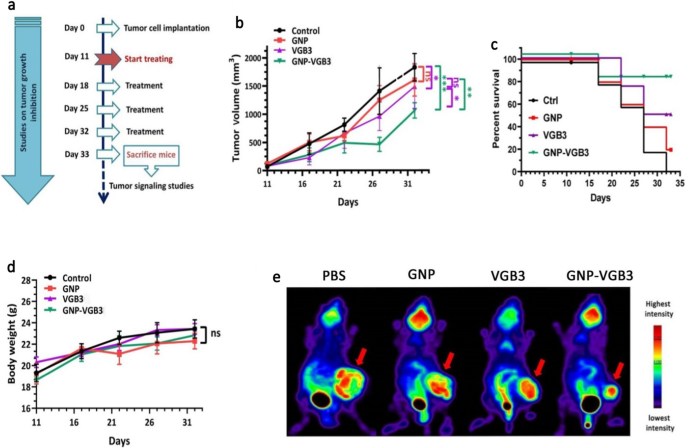
To find out whether or not the superior in vitro efficiency of GNP–VGB3 over free peptide and GNP is recapitulated in vivo, mice harboring 4T1 mammary carcinoma tumors, which is named a VEGF-dependent mannequin, had been handled with GNP–VGB3, GNP, free peptide or phosphate buffer saline (PBS) as management. When tumor measurement reached a median quantity of ~ 100 mm3, completely different remedy teams intravascularly (i.v.) injected as soon as every week for 3 weeks (Fig. 6a), and through this era, tumor quantity, physique weight and survival curve had been measured. On day 32, the typical tumor quantity within the GNP–VGB3-treated group (1062 mm3) was considerably decrease than in PBS (1828 mm3), GNP (1605 mm3), and free VGB3 (1485 mm3). These outcomes point out that tumor regression occurred in each VGB3 and GNP–VGB3-treated teams, however GNP–VGB3 was considerably more practical. Notably, GNP group confirmed no tumor development inhibition (Fig. 6b).
In vivo antitumor exercise of PBS, GNP, VGB3, and GNP–VGB3 in murine 4T1 mammary carcinoma tumor mannequin. a Schedule for animal experiments. b Tumor development inhibition; strains, imply tumor quantity for every group of six animals per group; error bars signify ± SEM. n = 6; **P < 0.01, ***P < 0.001; two-way ANOVA (c) The survival charges of 4T1-bearing mice after remedy had been illustrated by Kaplan–Meier curves., and d Physique weight measurements taken each 5 days till day 32, offered as imply ± SEM. All teams in contrast with management and analyzed by prism by two-way ANOVA statistical evaluation, imply ± SEM and n = 6 (***P < 0.001, **P < 0.01, ns: not important). e [18F]-fluorodeoxyglucose (18F-FDG) PET imaging of mice handled with GNP, VGB3, GNP–VGB3 or PBS at day 32. Consultant PET pictures are proven with arrows indicating 4T1 mammary carcinoma tumors
To additional assess the in vivo efficacy of remedies, animal survivals was adopted up within the remedy teams (n = 6) and the outcomes had been in contrast with PBS-treated controls. The survival curve deduced from Kaplan–Meier evaluation till the day 32 after implantation indicated that GNP–VGB3 (one mouse useless; 83.2% survival at day 32) extended the survival fee greater than VGB3 (two mice useless; 64% survival on the day 32) and GNP (4 mice useless; 16% survival at day 32) (Fig. 6c). All members of the management group had been misplaced earlier than day 32. As well as, the physique weight of all animals was elevated in the course of the remedy interval (Fig. 6d), suggesting that the remedies are unhazardous on the dosages used on this work.
To guage the impact of remedies on the tumor development in Balb/c mice, we carried out 18F-FDG-PET imaging on the finish of remedies (day 32). The FDG-PET pictures of Balb/c mice is offered in Fig. 6e. After remedy for 4 weeks (day 32), the imply uptake values of 18F-FDG was extra considerably decreased in GNP–VGB3 group than VGB3 group in contrast inside the group that obtained PBS, whereas the uptake worth didn’t change in GNP group. These outcomes supplied sturdy suggestive proof that the VGB3-mediated inhibition of tumor development is improved by gold nanoformulation.
Suppression of VEGFR-1/-2-mediated signaling in 4T1 mammary carcinoma tumors
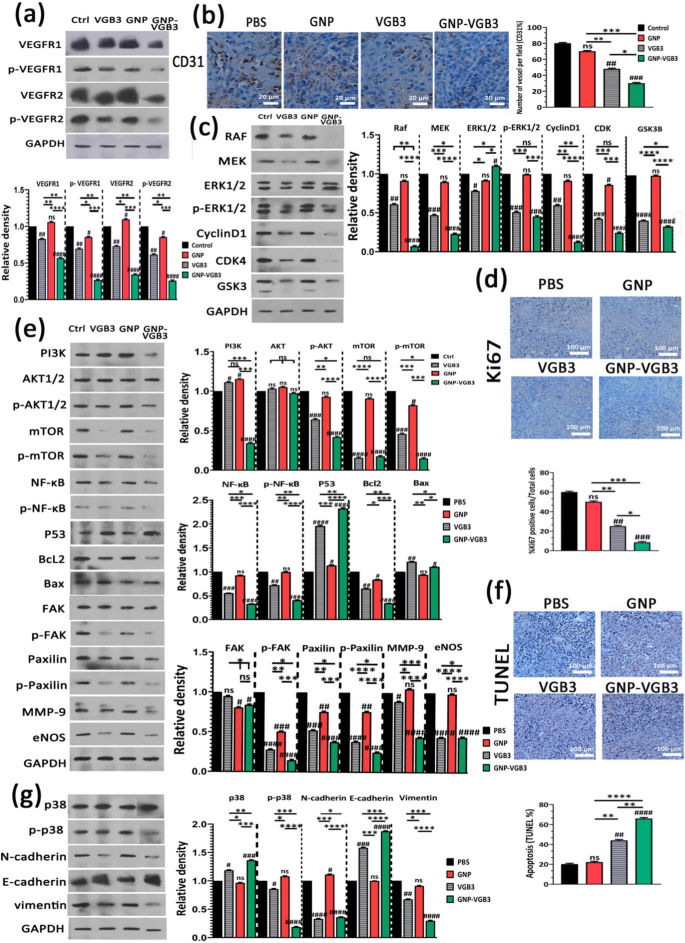
Our latest research demonstrated that VGB3/48 h is an efficient remedy for metastatic murine 4T1 mammary carcinoma tumors [6] by way of the inhibition of tumor cells proliferation (decreased Ki-67 expression), angiogenesis (decreased expression of CD31 and CD34), and the induction of apoptosis in tumors (elevated TUNEL staining and p53 expression, and decreased Bcl-2 expression) [13]. Outcomes of the present research revealed that weekly remedies attenuate the peptide efficacy, however conjugation to GNP considerably improved its antitumor properties. To discover the molecular mechanisms underlying the superior antitumor results of GNP–VGB3 in opposition to 4T1 mammary carcinoma tumors in contrast with free peptide, tumors had been harvested on the finish of the remedy interval (day 32 after implantation) and the VEGFR1/R2 signaling pathways had been assessed by western blot.
First, tumor lysates had been analyzed for whole and phosphorylated VEGFR-1 and VEGFR-2. VEGFR1 and VEGFR2 expressions had been ample in 4T1 tumors, and their expression ranges had been roughly equal in untreated tumors (Fig. 7a). This consequence, in accordance to our earlier investigations [6, 8, 9, 13], confirms that 4T1 mannequin is suitable for investigation of responses to VEGFR1 and VEGFR2 inhibition. Clearly, VEGFR1 and VEGFR2 expression ranges had been far more successfully inhibited when weekly handled by GNP–VGB3 (P < 0.0001) than VGB3 (P < 0.01) and GNP in comparison with controls.
Inhibition of VEGFR1/2-mediated signaling after remedy with PBS, GNP, VGB3 or GNP–VGB3 in 4T1 mammary carcinoma tumor-bearing mice. All the measurements had been accomplished on the finish of the remedy interval (day 32). a Tumor lysates had been probed and quantitatively analyzed for ranges of whole and phosphorylated VEGFR1 and VEGFR2. b Consultant pictures and quantitative evaluation of CD31 as microvessel formation index (Scale bar = 20 µm). c Tumor lysates had been probed and quantitatively analyzed with the indicated antibodies. d Consultant pictures and quantitative evaluation of Ki67 as tumor proliferation index (Scale bar = 100 µm). e Tumor lysates had been probed and quantitatively analyzed with the indicated antibodies. f Consultant pictures of TUNEL and the statistical graph primarily based on the share of apoptosis cells (Scale bar = 100 µm). g Tumor lysates had been probed and quantitatively analyzed with the indicated antibodies. All information had been analyzed by prism software program (One-way ANOVA technique, imply ± SD, n = 3, quantity signal image (#) was used for evaluating the remedies with management, and asterisk image (*) was used for comparability between remedies ****P < 0.0001, ***P < 0.001, **P < 0.01,*P < 0.1, ns: not important in comparison with untreated management)
Clearly, blockade of VEGFR1- and particularly VEGFR2-mediated signaling attenuates angiogenesis. To evaluate whether or not the antitumor impact of GNP–VGB3 is related to the inhibition of angiogenesis, the microvascular density (MVD) was quantified by immunohistochemical staining of CD31, as an index of angiogenesis. In comparison with PBS-treated management group, MVD was considerably diminished in VGB3-treated tumors (P < 0.01) (Fig. 7b); nonetheless, the best discount was noticed within the GNP–VGB3 group (51% discount, P < 0.001) in comparison with controls. In GNP group, there was no important discount in MVD (P > 0.9999).
The VEGFR-2 and, to a lesser extent, VEGFR1 has been proved to mediate varied mobile sign transduction, together with endothelial and tumoral cell survival, proliferation, migration, and induction of permeability [3]. Due to this fact, the implications of VEGFR-1/-2 blockade on the constitutive and phosphorylated types of widespread downstream proteins had been assayed by immunoblotting of the tumor lysates.
The RAS/RAF/MEK/ERK signaling pathway is essential for the regulation of various mobile processes, together with cell proliferation, differentiation and migration. Via cyclin D1 and cyclin-dependent kinases (Cdk)-2/-4, RAS/RAF/MEK/ERK pathway is concerned within the regulation of cell cycle development. We investigated the consequences of various remedies on this pathway by measurement of the expression ranges of RAF, MEK, CyclinD1, CDK and constitutive and phosphorylated types of ERK1/2. The outcomes confirmed that GNP is usually ineffective on these signaling mediators. Moreover, GNP–VGB3 resulted in downregulation of RAF, MEK, cyclinD1, CDK-4 and phosphorylated type of ERK1/2 extra potently than VGB3, which point out that the superior antitumor impact of GNP–VGB3 than free peptide is related to more practical inhibition of proliferation signaling. In settlement with these outcomes, immunohistochemical staining of Ki-67, an index tumor cell proliferation, was decreased by GNP–VGB3 (P < 0.001) extra potently than VGB3 (P < 0.01) in comparison with controls (Fig. 7d). Moreover, suppression of proliferation signaling supported by decreased expression of glycogen synthase kinase-3 (GSK-3), an inhibitor of cyclin D1, in VGB3- and GNP–VGB3-treated tumors (P < 0.0001) in comparison with controls (Fig. 7c).
Supporting cell survival and inhibition of apoptosis is one other consequence of RAS/RAF/MEK/ERK signaling. Furthermore, VEGF promotes cell survival, most cancers growth in addition to metastasis through the PI3K/Akt/mTOR signaling pathway [42]. Accordingly, we sought to additional examine the consequences of remedies on the survival, apoptosis and metastasis by evaluation of PI3K, AKT, p-AKT, mTOR and p-mTOR, NF-κB, p-NF-κB, P53, Bcl2 and Bax. In comparison with controls, the expression stage of PI3K elevated after remedy with GNP and VGB3 (P < 0.05) however markedly decreased after GNP–VGB3 remedy (P < 0.0001) (Fig. 7e). In settlement with these outcomes, GNP–VGB3 potently diminished p-AKT formation in 4T1 tumors (P < 0.0001), whereas VGB3 was much less efficient (P < 0.001) and GNP had no impact. mTOR often known as promoter of tumor cell migration and invasion [43]. GNP couldn’t change the expression stage of mTOR and p-mTOR. In distinction, each VGB3 and GNP–VGB3 remedies drastically suppressed whole mTOR expression (P < 0.0001). Moreover, phosphorylation of mTOR was inhibited extra successfully by GNP–VGB3 (P < 0.0001) than by free VGB3 (P < 0.001) in comparison with PBS-treated tumors. A serious goal of Akt is the NF-κB pathway [44]. Evaluation of tumors revealed extremely important lower within the expression of NF-κB in VGB3- and GNP–VGB3-treated tumors (P < 0.001 and P < 0.0001, respectively). Extra importantly, GNP–VGB3 resulted in sturdy suppression of NF-κB phosphorylation, whereas GNP had no impact and VGB3 group modestly confirmed NF-κB phosphorylation. Provided that activation of NF-κB results in blockade of apoptosis and promotion of cell proliferation [45], downregulation of NF-κB and p-NF-κB is anticipated to induce apoptosis in tumors. Accordingly, VGB3-treated tumors current with a lot greater P53 ranges than management 4T1 tumor lysates (P < 0.0001); nonetheless, P53 much more elevated upon administration of GNP–VGB3 (Fig. 7e). In parallel, GNP–VGB3 remedy resulted in a lower Bcl2 expression with a concomitant enhance within the protein stage of Bax (Fig. 7e). These information, in line with ROS overproduction and annexin V staining in HUVE and 4T1 cells, counsel that GNP–VGB3 enhanced the VGB3-driven inhibition of survival signaling, resulting in apoptosis induction in 4T1 mammary carcinoma tumors. To corroborate these outcomes, tumors had been analyzed with the TUNEL apoptosis assay. Notably, whereas VGB3 remedy alone had a low impact on the TUNEL-positive cells (P < 0.01), the conjugation of VGB3 to GNP appeared to strengthen the apoptosis induction property of the VEGFR1/2-blocking peptide, as evidenced by the marked rising of the TUNEL-positive tumor cells in GNP–VGB3-treated tumors (P < 0.0001) (Fig. 7f).
FAK/Paxillin signaling axis, in downstream of PI3K/AKT and MAPK/ERK signaling pathways, is concerned in cell adhesion, migration, proliferation, and survival. Evaluation of tumor lysates revealed a reasonable discount of whole FAK for each VGB3 and GNP–VGB3-treated tumors (P < 0.05) whereas Paxillin expression was significantly decreased in VGB3 and GNP–VGB3-treated tumors in comparison with management (P < 0.001 and P < 0.0001, respectively). Extra strikingly, p-FAK in addition to p-paxillin formation had been strongly inhibited by all remedies (P < 0.0001) in order that GNP–VGB3 and GNP had been essentially the most and fewer efficient teams, respectively. These outcomes are indicative of extra environment friendly suppression of cell detachment, as an preliminary step within the metastatic transformation, by GNP–VGB3 than by different remedies (Fig. 7e).
The method of cell invasion is a mixture of cell migration with concurrent degradation of the encircling extracellular matrix (ECM) by matrix metalloproteases [46]. Decreased expression of MMP-9 has been noticed in 4T1 mammary carcinoma tumors handled with VEGF blockading peptides [9]. Importantly, GNP–VGB3 potently inhibited MMP-9 expression in tumor tissue (P < 0.0001), whereas free peptide was reasonably efficient (P < 0.05) and GNP had no impact (Fig. 7e).
Inhibition of VEGFR2 phosphorylation was proven to inhibit metastasis and most cancers development through eNOS/Akt signaling [47], underlined by the truth that endothelial nitric oxide synthase (eNOS) induces nitric oxide (NO) manufacturing, which performs necessary position in vascular safety, focal adhesion formation and cell migration. Our outcomes indicated that GNP-treatment has no impact on eNOS expression in tumor tissues, whereas each VGB3 and GNP–VGB3 remedies markedly suppressed eNOS expression in comparison with controls (P < 0.0001) (Fig. 7e).
p38 MAP kinase has been implicated in a wide range of mobile processes, together with cell proliferation, cell differentiation, apoptosis, cell migration, and invasion [48, 49]. The entire expressions of p38 MAPK had been unaffected by GNP remedy however elevated by VGB3 (P<0.05) and GNP–VGB3 (P < 0.001) in comparison with controls. Extra strikingly, p38 MAP kinase activation, i.e. p-p38 MAP kinase formation, strongly suppressed by GNP–VGB3 (P < 0.0001) however not by the opposite remedies in comparison with controls (Fig. 7g).
Epithelial–mesenchymal transition (EMT) promotes metastasis by enhancing mobility, invasion, and resistance to apoptotic stimuli [50]. Importantly, EMT is characterised by decreased expression of cell adhesion molecules comparable to E-cadherin and elevated expression of vimentin and N-cadherin. We due to this fact in contrast the potential of remedies to have an effect on the expression of E-cadherin, vimentin and N-cadherin. GNP–VGB3 remedy was more practical than VGB3 in reducing the expression of vimentin and N-cadherin in addition to in rising the expression of E-cadherin in 4T1 tumors (P < 0.0001), whereas the expression ranges of each proteins in GNP-treated tumors had been comparable with controls (Fig. 7g). These outcomes point out that GNP–VGB3 attenuated EMT in 4T1-bearing Balb/c mice.
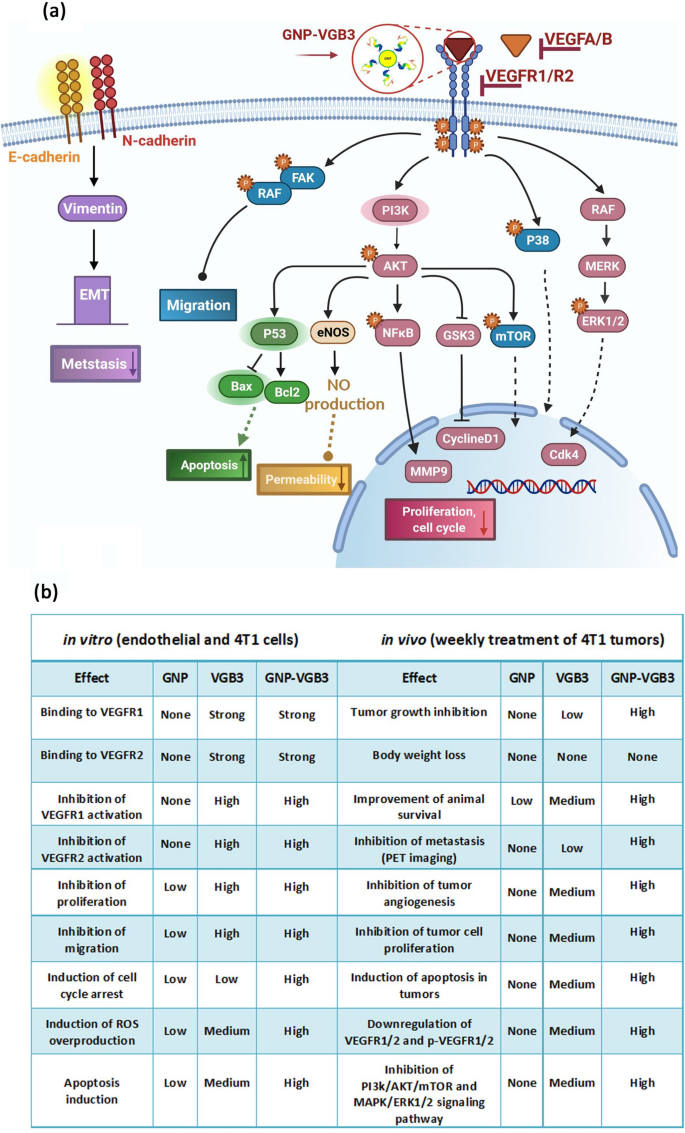
Though most of identified responses to VEGFA are mediated by VEGFR2, endothelial cell features could be stimulated by VEGFR1 particularly by way of PI3K/Akt pathway [51]. In settlement with the in vitro outcomes, evaluation of signaling pathways in tumor tissues confirmed that even larger inhibitory results than these resulted from VEGFR1/VEGFR2 blockading peptide could be obtained from its mixture with the constructive results of GNP. Determine 8 represents the large ranges of signaling mediators focused by GNP–VGB3.
Signaling transduction and organic processes mediated by inhibition of VEGFR1 and VEGFR2 utilizing GNP–VGB3. a Schematic illustration of the impact of GNP–VGB3 on the downstream signaling pathways of VEGFR1/2. Binding of GNP–VGB3 to VEGFR1/R2 inhibits the exercise of receptors that had been activated by VEGFA/B and the downstream signaling pathways is subsequently prevented. VGB3 after conjugation to GNPs suppresses the signaling pathways in through stopping cell proliferation, migration, apoptosis, permeability and metastasis. b The comparability of in vitro and in vivo results exerted free GNP, free VGB3 peptide and GNP-VGB3
MicroCT imaging
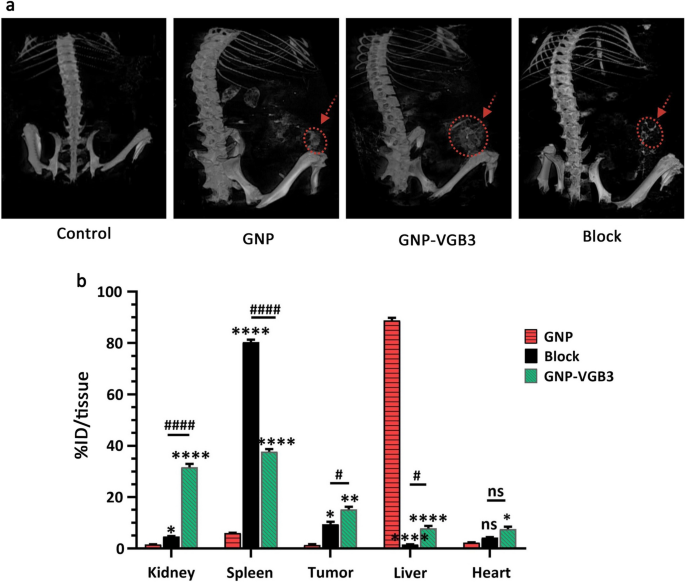
To determine the buildup of nanoparticles within the tumors, we examined mice handled with the GNP, VGB3 and GNP-VGB3 utilizing MicroCT imaging. Initially, one group of 4T1 tumor-bearing Balb/c mice (n = 3) had been intravenously injected with free GNPs. After injection for 3 h, the strongest CT alerts had been noticed in kidneys (Fig. 9a). As well as, CT alerts had been noticed in tumors and liver (Fig. 9a). Nonetheless, the alerts within the tumor areas had been augmented in mice handled with peptide certain GNP (GNP–VGB3) (Fig. 9a). Moreover, goal specificity of GNP–VGB3 elicited by a blocking experiment. As proven in Fig. 9a, accumulations had been suppressed successfully by coadministration of competing free VGB3 peptide. These observations counsel that GNP–VGB3 can particularly goal mammary carcinoma tumors.
Biodistribution research by MicroCT imaging and ICP MS evaluation. a Consultant 3D-reconstructed whole-body CT pictures of mice bearing 4T1 tumors at 3 h following intravenous (i.v.) injection of GNP, GNP–VGB3, block and PBS (untreated management). The pink circles and arrows point out tumor places. b The Au concentrations in main organs, together with kidney, spleen, tumor, liver and coronary heart, which obtained GNP, GNP–VGB3 and block samples quantified after 24 h utilizing inductively coupled plasma mass spectrometry (ICP-MS) evaluation. The quantified outcomes had been outlined primarily based on the share of injection dose per tissue (%ID/tissue) and analyzed statistically by two-way ANOVA technique (imply ± SEM, and n = 3, ****P < 0.0001, **P < 0.01, *P < 0.05, or ns: not important). (Asterisk image (*) was used for comparability between GNP with GNP–VGB3 and block and quantity signal image (#) was used for evaluating GNP–VGB3 and block)
Biodistribution research of nanoparticles
Quantitative biodistribution evaluation was carried out by inspecting the gold content material (% ID/tissue). To this finish, the mice had been sacrificed, a sequence of dissected organs (kidney, spleen, liver and coronary heart) and tumor tissues of mice (n = 3) had been freshly collected 24 h post-injection, and inductively coupled plasma mass spectrometry (ICP-MS) measurements had been instantly taken. As proven in Fig. 9b, a really excessive quantity of gold was present in liver (88.8% ID/tissue) and spleen (80.3% ID/tissue) for GNP and blocking teams (n = 3), respectively, suggesting that these nanoparticles are cleared primarily by way of the reticuloendothelial system. In these teams, the tumor accumulations had been 1.3 and eight% ID/tissue, respectively. The tumor accumulation of GNP–VGB3, nonetheless, elevated to fifteen.2% ID/tissue, which was considerably greater than these of GNP (1.4%) and blocking (9.4%). Importantly, the very best accumulation of GNP–VGB3 was noticed in kidney. The remark that free GNP is accrued principally within the liver was additionally reported in earlier investigations [52, 53]. Nanosystems with renal clearance are extra fascinating than these cleared by way of the reticuloendothelial system [54]. Thus, greater accumulation of GNP–VGB3 kidney could induce much less injury than free GNP within the regular tissues. These information counsel that the tumor accumulation of GNP–VGB3 is extra environment friendly than free GNP. As well as, a decreased quantity of GNP–VGB3 within the presence of free peptide (block) additional confirms its particular binding to tumors.